Microbiome and Clinical Physiology
“Our goal is to understand host-microbiota-diet interactions to develop targeted therapies that prevent and treat human diseases and promote healthy aging”.
DR. RAFAEL VALDÉS MAS RESEARCHER. MICROBIOME AND CLINICAL PHYSIOLOGY RESEARCH GROUP
Over the past twenty years, microbiome research has flourished, revealing that the trillions of microorganisms living in and on the human body play essential roles in health. Bacteria, viruses, fungi, and parasites influence metabolic and immune functions, not only in the gut but also in distant organs. Their interactions can either drive disease development or help maintain physiological balance, highlighting their profound impact on human biology.
To explore the intricate interactions between the diverse microorganisms in the human body and their connection to human biological processes, we utilize multi-omics methodologies combined with advanced computational algorithms. Our findings are validated through mouse model experiments, and organoid culture techniques. Ultimately, our goal is to translate these discoveries from the laboratory to clinical applications through human studies.
Currently, a key focus of our research is understanding the role of the microbiome and diet in cancer, healthy aging, and systems nutrition.

Dr. Rafael Valdés Mas
GROUP LEADER
| +34 948 194 700 | |
| rvaldesmas@unav.es | |
| Research profile |
Our main lines of work
Novel computational approaches
Develop novel computational approaches to integrate multi-omics data and characterize the complex interactions between the host, microbiome, and diet.
Microbiome and human diseases
Uncover microbiome-driven mechanisms influencing human diseases and aging processes.
Design therapies
Design microbiome- and diet-based targeted therapies for disease prevention and treatment.
HIGHLIGHTS
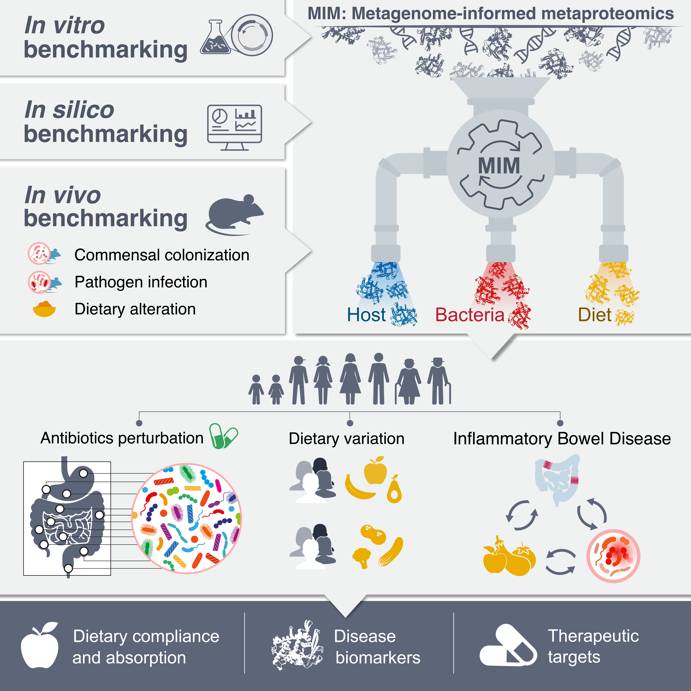
Metagenome-informed metaproteomics (MIM) is our novel revolutionary methodology that enables simultaneous functional profiling of diet, host, and microbiome, offering unprecedented insights into disease mechanisms. By distinguishing compositional from functional dysbiosis, MIM uncovers microbial and host-driven alterations across various disease contexts. In inflammatory bowel disease (IBD), it reveals how host immune cues suppress commensal microbes and identifies trans-kingdom biomarkers with diagnostic potential. Moreover, MIM provides a quantitative assessment of diet-related shifts in IBD, evaluating exclusive enteral nutrition (EEN) compliance and detecting small intestinal malabsorption, positioning it as a powerful tool for personalized medicine and microbiome-targeted therapies.
Meet the research team



Scientific activity of the Microbiome and Clinical Physiology Research Group
Latest scientific publications
- Metagenome-informed metaproteomics of the human gut microbiome, host, and dietary exposome uncovers signatures of health and inflammatory bowel disease Jan 20, 2025 | Magazine: Cell
- Phage therapy in noncommunicable diseases Oct 20, 2023 | Magazine: Science
- The microbial genotoxin colibactin exacerbates mismatch repair mutations in colorectal tumors Sep 14, 2023 | Magazine: Neoplasia
- Epithelial Nlrp10 inflammasome mediates protection against intestinal autoinflammation Apr 24, 2023 | Magazine: Nature Immunology
